Lea en español
As the COVID-19 pandemic continues to affect patients across the nation, clinical treatment trials have been critical to identifying new potential treatments. A group of University researchers have taken up a novel project which attempts to analyze demographic disparities within these trials themselves, and devise fruitful solutions for increasingly equitable healthcare.
Asst. Medicine Prof. Kathleen McManus collaborated with researchers from other institutions to investigate how access to these trials has differed across different demographics and different areas of the U.S. Clinical treatment trials are the most effective method of testing potential treatments, and running a trial accessible for all demographic groups encourages equitable health practices.
Additionally, lead researcher Rohan Khazanchi explained, COVID-19 has affected different demographics with varying degrees of severity and symptoms, so organizing a diverse participant pool would demonstrate a treatment’s differing effects.
“It's important to think about what patient population is included in a clinical trial because it helps you understand who that intervention that is being studied could actually help,” Khazanchi said.
Clinical treatment trials are often expensive and can therefore only be run by institutions that are well-resourced and well-funded, collaborating researcher Elizabeth McQuade noted in greater detail. Since urban academic institutions tend to have better funding, patients living in rural areas are less likely to have access to treatment trials.
“They have to have the right Institutional Review Board setup, they have to have the clinical staff infrastructure available to put up the trial,” McQuade said. “So, it generally happens in these larger medical centers, often conducted in main cities.”
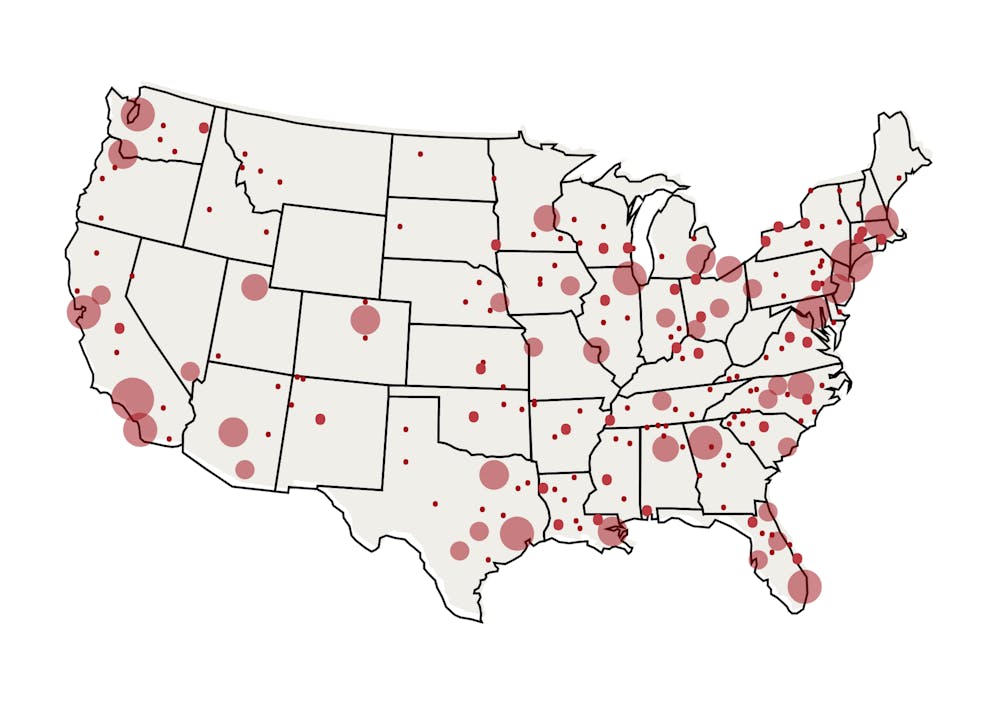
There are currently 87 COVID-19 clinical trials run in Virginia at institutions such as U.Va. Health, Virginia Commonwealth University and Inova Health System.
The research team, including McManus, Khazanchi, and McQuade, highlighted differences in access to trials by comparing driving times to therapeutic trial sites. They focused on COVID-19 treatment trials that began between Jan. 20 and Sept. 20, 2020 and identified the trial site closest to the center of population for each census district by driving time. They then quantified representation by calculating the proportions of demographic subgroups living within these census districts using information from the 2015-2019 U.S. Census American Community Survey.
After analyzing their data, the team uncovered two main statistically significant disparities in clinical trial access.
American Indian and Alaskan Native populations appeared to face longer driving times even when accounting for rurality, with over 50 percent of these individuals living over an hour away from the nearest trial site. Conversely, while Black and Hispanic populations were found to live in relatively close proximity to COVID-19 therapeutic trials, past research shows they are nevertheless underrepresented in treatment trials and face hospitalization at higher rates. Moreover, 31.3 percent of U.S. residents live over an hour away from their closest site.
Since most other treatment trials can only run in well-resourced and well-funded hospitals as well, the team’s research exposed inequities in treatment trial access and participant recruitment that may be prevalent across clinical research, lead researcher Khazanchi said.
“Our goal wasn't to make causal inference,” Khazanchi said. “Our goal was really to highlight that this is an issue.”
These calculated disparities in access to trials do not numerically explain for even lower participation and recruitment actually observed among American Indian, Alaskan Native, Black, and Hispanic populations, researchers found. Socioeconomic inequities that impact barriers to transportation could additionally impact actual participation in clinical treatment trials, while the methodology solely accounted for driving time, as Khazanchi noted.
“That's especially important in very rural census tracts,” Khazanchi said. “We may underestimate some of these differences, as well as in urban areas that are really close to trial sites but may have other reasons why access is poor.”
Additionally, there may be cultural barriers that discourage certain residents from participating in clinical trials, or researchers from recruiting patients, as McQuade explained.
“To be part of a trial, you have to have informed consent which requires a long conversation,” McQuade said. “So you can imagine how that would reduce enrollment of individuals that don't speak English as a first language.”
These findings lead to questions about these disparities and how they can be considered as future clinical trial approaches.
While increasing funding for rural academic institutions in order for more rural areas to have access to trials would require broad measures, current patient recruitment methods and clinical trial formats themselves could be modified to mitigate this. One approach is to delocalize clinical treatment trials through online or remote clinical trials.
Online and remote trials would allow treatments to be mailed directly to patients, regardless of their location, bypassing the effects of aggregated trials in urbanized areas. Two University researchers’ recent work led to an ongoing remote clinical trial led by researchers at Washington University in St. Louis.
These researchers — Assoc. Neuroscience Prof. Alban Gaultier and University researcher Dorian A. Rosen — researched the antidepressant fluvoxamine’s benefits in suppressing the inflammatory response sepsis, a life-threatening extreme response of the immune system sometimes present in COVID-19 patients. In order to test the effectiveness of fluvoxamine, the researchers first ran a traditional clinical trial with preliminary success with local St. Louis residents, then launched a remote trial open to residents across the U.S. and Canada.
A remote trial format utilizing devices that are not dependent on the Internet could help further ease the effects of socioeconomic and racial disparities.
“By doing this, you can reach anybody regardless of their socioeconomic status, access to transportation, access to Internet … and you can reach people in rural communities who are often not included,” Gaultier said.
A remote or online clinical trial cannot correct all disparities and theoretically would not be fit for all medical treatments, such as those that may induce urgent medical situations dependent on nearby hospitals, Gaultier said.
“The COVID trial went beautifully, because of people who were doing PCR tests,” Gaultier said. “If you have a brain tumor or some autoimmune disorder … then the remote format might not be the best.”
While remote trials would not resolve all of the disparities affecting clinical trial recruitment, the inclusivity of clinical trials themselves can perhaps be actively monitored because of policies on a larger scale. According to Khazanchi, there has been a greater incentive and effort for mandating this information following trials.
“We're starting to see a move towards … requiring when you publish manuscripts with clinical research data that you at least include a supplement or a table that kind of overviews the demographic composition of your population,” Khazanchi said.
Khazanchi and the collaborating researchers highlighted important inequities in access to COVID-19 treatment trials. However, these disparities provide the potential to continue improving treatment trial infrastructure to broaden access, and for the collaboration between researchers and the government to support the general population’s health.
“I think there's a real need for thought leadership on how we can reform clinical trials moving forward, and just demonstrating the feasibility and practicality of making some of those interventions,” Khazanchi said.