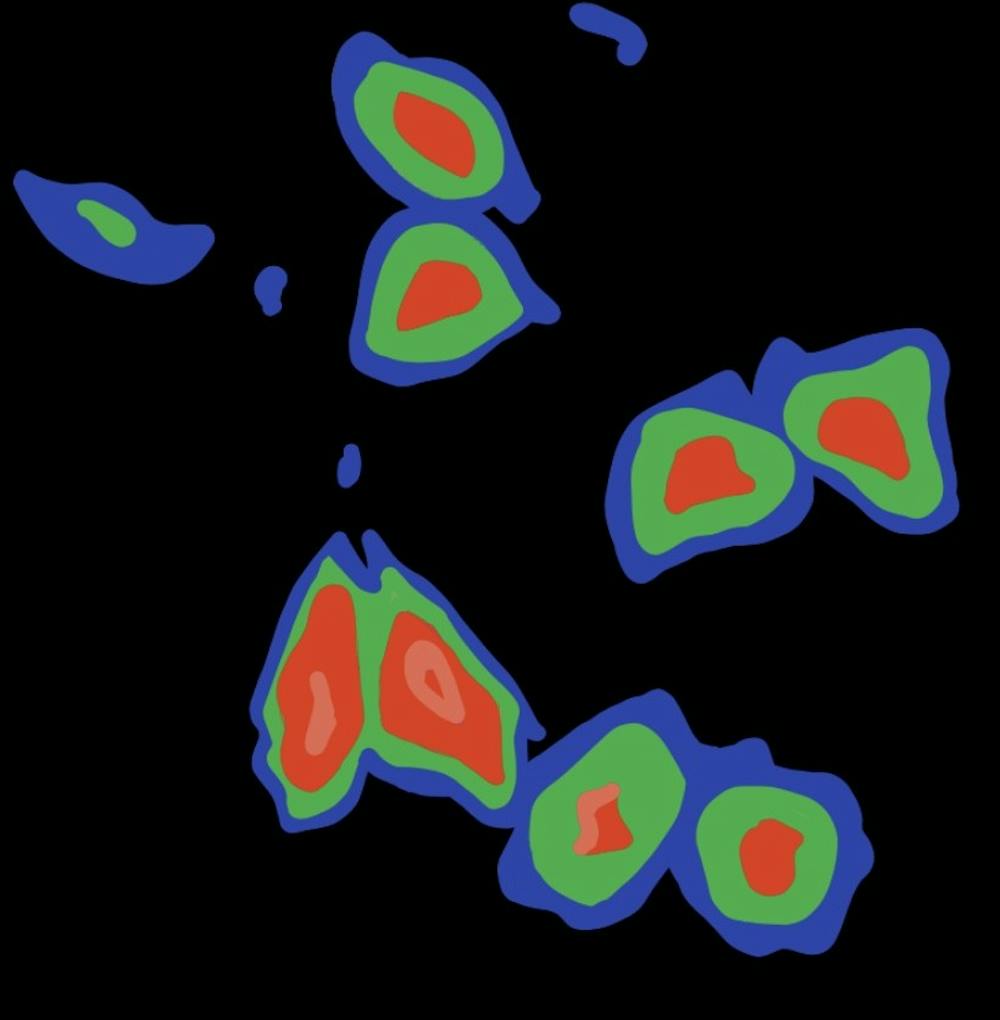
Fluorescent biosensors are powerful research tools, allowing scientists to study metabolic processes in individual cells or visualize chemical signaling networks. These biosensors traditionally emit green or yellow light, although red biosensors inherently have more useful properties. Two researchers in the School of Medicine recently discovered a convenient and efficient method to red-shift fluorescent protein-based biosensors using the amino acid 3-aminotyrosine.
Prior to the publication of this study, existing methods to convert green or yellow fluorescent protein-based biosensors to red were partially successful or excessively difficult, requiring special conditions or additional electron donors. Now, this finding allows researchers in a wide breadth of disciplines — including biology, immunology and neuroscience — to rapidly red-shift biosensors for their own research needs.
For Dr. Hui-wang Ai, associate professor in the department of Molecular Biology and Biological Physics, this serendipitous discovery had been six years in the making. Ai was working alongside Dr. Shen Zhang, a post-doctoral researcher in the Ai Lab, on an unrelated project involving biosensors at the time of this finding.
“It wasn’t until one day when one of our previous lab members and I tried to incorporate this amino acid, 3-aminotyrosine, to the chromophore of a random green fluorescent protein,” Zhang said. “It turned out to have a huge red shift. That was totally unexpected at the time.”
The use of 3-aminotyrosine as an efficient mechanism to red-shift fluorescent biosensors became even more promising when Ai and Zhang noticed their red biosensors had advantageous properties that biosensors shifted through that other techniques did not possess.
Their biosensors required little optimization after being modified, meaning they maintained the brightness and responsiveness of the green biosensors while simultaneously adopting the favorable qualities associated with red color.
“[The red fluorescent biosensors] all retained their performance compared to the green version,” Zhang said. “They also have several advantages over green fluorescent biosensors. Red color features low phototoxicity and deeper tissue penetration.”
After observing these properties in one red-shifted biosensor, Ai and Zhang successfully red-shifted seven more fluorescent biosensors using 3-aminotyrosine. In test experiments, all eight red-shifted biosensors exhibited these advanced features and were used to sense ions, compounds and neurotransmitters like calcium or dopamine. These findings suggest that this method could be used to red-shift any fluorescent protein biosensors.
“Eventually, we showed that this is a generalizable strategy,” Ai said. “In theory, we can apply this to any biosensor — not only existing ones, but maybe even future ones.”
Before publishing their findings, Ai and Zhang applied this method to examine the metabolic dynamics of pancreatic beta cells using red-shifted fluorescent biosensors.
“For this particular paper, we examined some of the metabolic signaling molecules after glucose increased in the beta cells,” Ai said. “We found a phenomenon which could potentially be a new mechanism for beta cell signaling with glucose-induced insulin secretion.”
Beta cell signaling, which helps regulate blood sugar, is relevant in understanding diabetes at a molecular level. These findings about such signaling — facilitated by the improved spatial and temporal resolution of red-shifted biosensors — are a first glimpse into one of many positive applications this discovery could have.
Ai and Zhang later published this research in a well-known scientific journal, Nature Chemical Biology, so that other scientists can harness the efficiency and power of 3-aminotyrosine to red-shift biosensors.
Importantly, they noted, this method is convenient to the average research scientist.
“The non-canonical amino acid [3-aminotyrosine] is pretty easy to access,” Zhang said. “You basically just buy it from different companies.”
In addition to being accessible, this method to red-shift fluorescent biosensors can be applied to examine a diverse range of scientific processes.
“Through monitoring the fluorescence intensity change, [you] are able to monitor the dynamics of … [your] analyte of interest,” Zhang said.
The relevance of red-shifted biosensors suggests that this discovery will have a widespread impact on different domains of science and research. Examples of applications include using biosensors to examine solutes in the blood or track the effect of behavioral changes through serotonin levels in the brain.
Within their own research, Ai and Zhang eventually hope to apply this discovery to extend fluorescent biosensors beyond the cellular level into model organisms such as fruit flies or mice.
For now, they are working with other researchers at the University to use biosensors to better understand clinical therapies such as pancreatic islet transplantation.
Pancreatic islet cells — which are also implicated in beta cell signaling and blood sugar regulation — produce hormones like insulin and glucagon that play an important role in the pathology of diabetes.
“In one example, my lab is collaborating with medical doctors doing islet transplantation,” Ai said. “We are trying to use these biosensors to evaluate islet quality before transplantation to potentially advance medical therapy for diabetes. This could really be a trend for the future.”