The Food and Drug Administration on March 28 approved the first drug to treat primary progressive Multiple Sclerosis, offering a promising treatment option to patients who previously had few options in coping with the unpredictable, disabling disease.
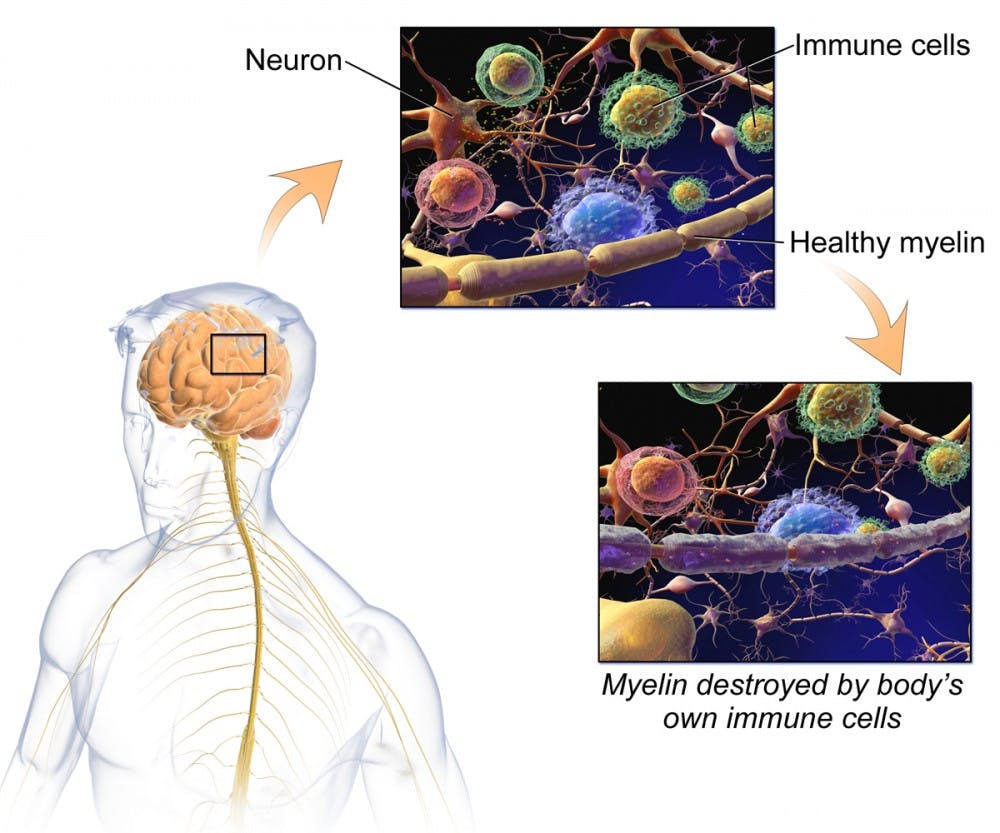
The drug, ocrelizumab, under brand name Ocrevus, targets specific types of B lymphocytes, a type of immune cell. While B lymphocytes represent a pivotal part of the immune system, they can malfunction and attack myelinated neurons, triggering MS. Ocrevus aims to reduce the frequency of these attacks.
“I think that Ocrevus will become a major player in the MS therapeutic arena,” said David Jones, neurologist at the James Q. Miller Multiple Sclerosis Clinic and asst. prof. of neurology. “I think it has very strong efficacy. The safety profile as we understand it now looks very good.”
MS is a complex inflammatory disease of the central nervous system. While the cause of the disease remains unknown, there are a variety of immunologic and environmental factors thought to increase risk. Over 200 genetic loci were identified in increasing the risk of the disease. Since the disease is most common in Northern Europeans, individuals with lower levels of vitamin D are thought to have particularly serious symptoms.
“There is a geographic component to the disease as it seems to affect those further away from the equator more than those close to it,” said Peggy Scott, nurse care coordinator for the James Q. Miller Multiple Sclerosis Clinic.
There are four types of MS — clinically isolated syndrome (CIS), relapsing-remitting MS (RRMS), primary progressive MS (PPMS) and secondary progressive MS (SPMS). CIS refers to the first episode of neurologic symptoms caused by inflammation and demyelination of the central nervous system. Eighty-five percent of MS patients are first diagnosed with RRMS — in which there is a neurologic symptom lasting for at least 24 hours before improving.
After about 15 years, patients with RRMS transition to SPMS — their condition worsens gradually over time. PPMS, however, is characterized by worsening neurologic function from the onset of symptoms, without early relapses or remissions. Prior to Ocrevus, the only FDA approved medications for MS only treated RRMS.
There are several disease-modifying therapies available to treat RRMS. However, because the cause of MS remains unknown, drugs can only aim to reduce future symptoms of the disease and delay disability.
“You can imagine that it would lead to somewhat of a challenge with MS patients, when the doctor may be saying ‘I want to prevent you from getting worse’ and the patient says, ‘I want to feel better today,’” Jones said. “Without understanding, patients may be disappointed in the drug and the care they are receiving, and sometimes may stop taking the medication, which may then lead to worsening.”
The FDA approved Ocrevus to treat both PPMS — the first drug for this type of MS — and RRMS. In fact, Ocrevus showed the most notable results in patients with RRMS, as the disease progression appeared to stop with no major side effects. In patients with PPMS, Ocrevus slowed — but did not stop — patients’ decline.
Apart from DMTs, managing other MS symptoms is also vital in helping individuals adapt to their disease and remain functional for as long as possible.
“A big issue is the silent symptoms of MS including fatigue, depression, cognitive impairment and pain,” Jones said. “People with MS are often told they look so good, yet do not feel so good and may struggle to meet the demands life puts on them.”
Jones said research for treating silent symptoms has been inadequate, and many doctors turn to ‘off-label’ use of medications or non-medication-based treatments such as healthy living and exercise.
Jones aims to keep MS patients from being defined by their disease.
“I want them to be people who just happen to have MS but are doing the things that they want to do,” Jones said. “Sometimes for MS patients, MS becomes the center of their universe, and then they really get into a vicious cycle that can be hard to break.”
Ocrevus costs less than many other DMTs on the market. It is administered intravenously twice a year and is viewed as relatively safe, revealing no serious side effects thus far. Ocrevus has only been studied and approved for adults, yet it may be an option for children with the disease in the future.
“As these newer drugs become available and are studied in the context of pediatric MS, more choices will thereby become available to our younger patients — but the first step towards this is always FDA approval in adult patients first,” said James Nicholas Brenton, pediatric neurologist in the Pediatric Neurology and Epilepsy Clinic and asst. prof. of medicine.
Correction: This article incorrectly stated the FDA approved Ocrevus on April 4. The drug was actually approved on March 28. This article has been updated with the correct date.